PK & Distribution
inner ear PK studies
Whatever the route of administration, the drug candidate for hearing loss or tinnitus has to reach its target, the inner ear, at the desired concentration. Cilcare’s PK studies allow the assessment of the bioavailability of the candidate in the inner ear, to determine its kinetic profile, and to estimate the best time-point and concentration for dosing in a subsequent efficacy study:
- Route of administration: local (transtympanic, intrabullar, intracochlear, round window niche) and systemic (PO, IP, IV, SC, IM)
- Kinetics after single (injection or infusion), or repeated administration
- Samplings: inner ear fluid, tympanic bulla, cerebrospinal fluid (CSF), different brain structures, blood & plasma
- Bioanalytical data on biological samples*:
- Exploratory LC-MS/MS analysis (small molecules)
- ELISA assays, commercial or customized
- Expertise in the processing of inner ear fluid & tympanic bulla tissue
*Performed by Cilcare’s partners
PK studies for other therapeutic indications
In addition to our expertise in hearing-related indications, Cilcare specializes in conducting pharmacokinetics studies in a wide range of therapeutic areas, with a particular focus on brain and central nervous system (CNS) diseases:
- Route of administration: PO, IP, IV, SC, IM, intrathecal
- Kinetics after single (injection or infusion), or repeated administration
- Samplings:
- Fluids: plasma, serum, CSF
- Organs:
- Liver, kidney, spleen, gonads, heart, lungs, vitreous humor of the eye
- Brain: whole brain, hippocampus, striatum, inferior colliculus, hypothalamus, cochlear nucleus, brainstem, frontal cortex, pituitary, any other structure
- Spinal cord (rat only)
- Bone (femur)
- Knee
vectors & gene therapy distribution
As biotherapy experts, we specialize in providing a range of assays to meticulously evaluate the biodistribution of gene therapy vectors, including AAV, and the effectiveness of gene therapy.
Assessing the biodistribution of viral vectors in gene therapy research traditionally relies on reporter gene expression, using fluorescent or luminescent signals to identify transduced tissues and cells. However, AAV vectors have limited DNA capacity, hindering the co-expression of therapeutic and reporter genes. Additionally, immune responses to fluorescent proteins like GFP can cause damage, especially in the central nervous system. Relying solely on reporter gene expression assumes equivalent transduction efficiency, which may not be accurate. To address these issues, it is essential to evaluate viral vector biodistribution directly, ensuring a more comprehensive understanding of their in vivo distribution.
Cilcare’s capabilities include, but are not limited to:
- Tissue processing: collection, fixation, embedding and sectioning
- H&E, immunohistochemistry, immunofluorescence
- Microscopy
- qPCR: quantification of the presence of the therapeutic gene in different tissues or organs
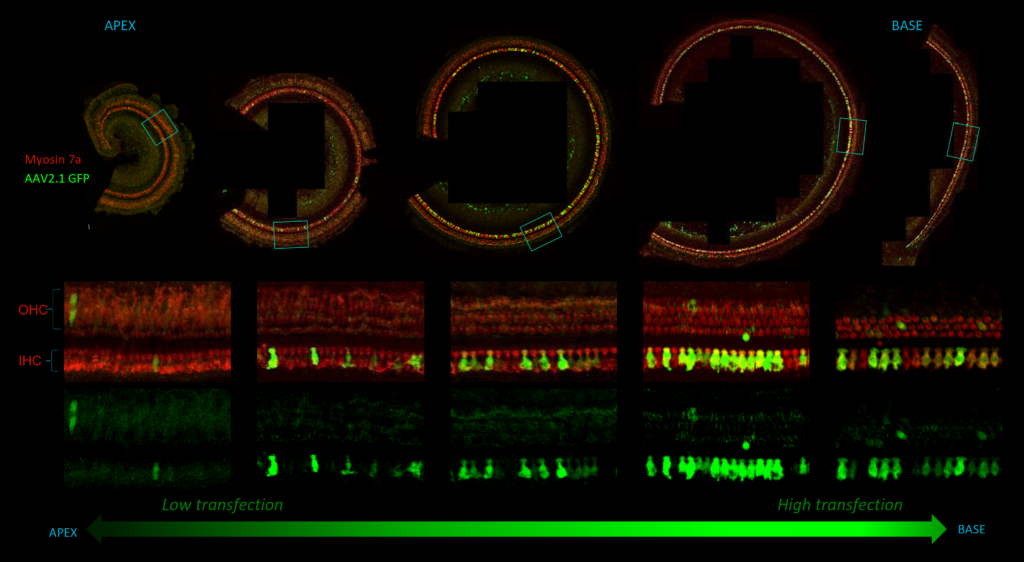
Distribution of an AAV transfection in the inner ear in guinea pig, from the apex to the base
biomarker studies
Cilcare’s comprehensive in vivo biomarker studies are tailored to deliver precise insight into the dynamics of biological processes within living organisms. Leveraging cutting-edge technologies and rigorous methodology, we offer a wide range of services designed to facilitate advanced research and development. Whether you’re exploring therapeutic interventions, investigating disease mechanisms, or seeking to optimize treatment strategies, our expertise in in vivo biomarker analysis provides invaluable support:
- Plasma-based: assessing liver and kidney toxicity by evaluating associated enzymes, signs of inflammation (complete blood count and C-reactive protein), metabolic indicators (glucose levels, glycated hemoglobin), and more.
- Transcriptomics: analyzing transcriptome expression (RNA sequencing) or specific gene expression (mRNA by q-RT PCR) in fluids or tissues.
- Proteomics: assessing protein expression in fluids or tissues using ELISA or multiplex methods (e.g., for inflammation).